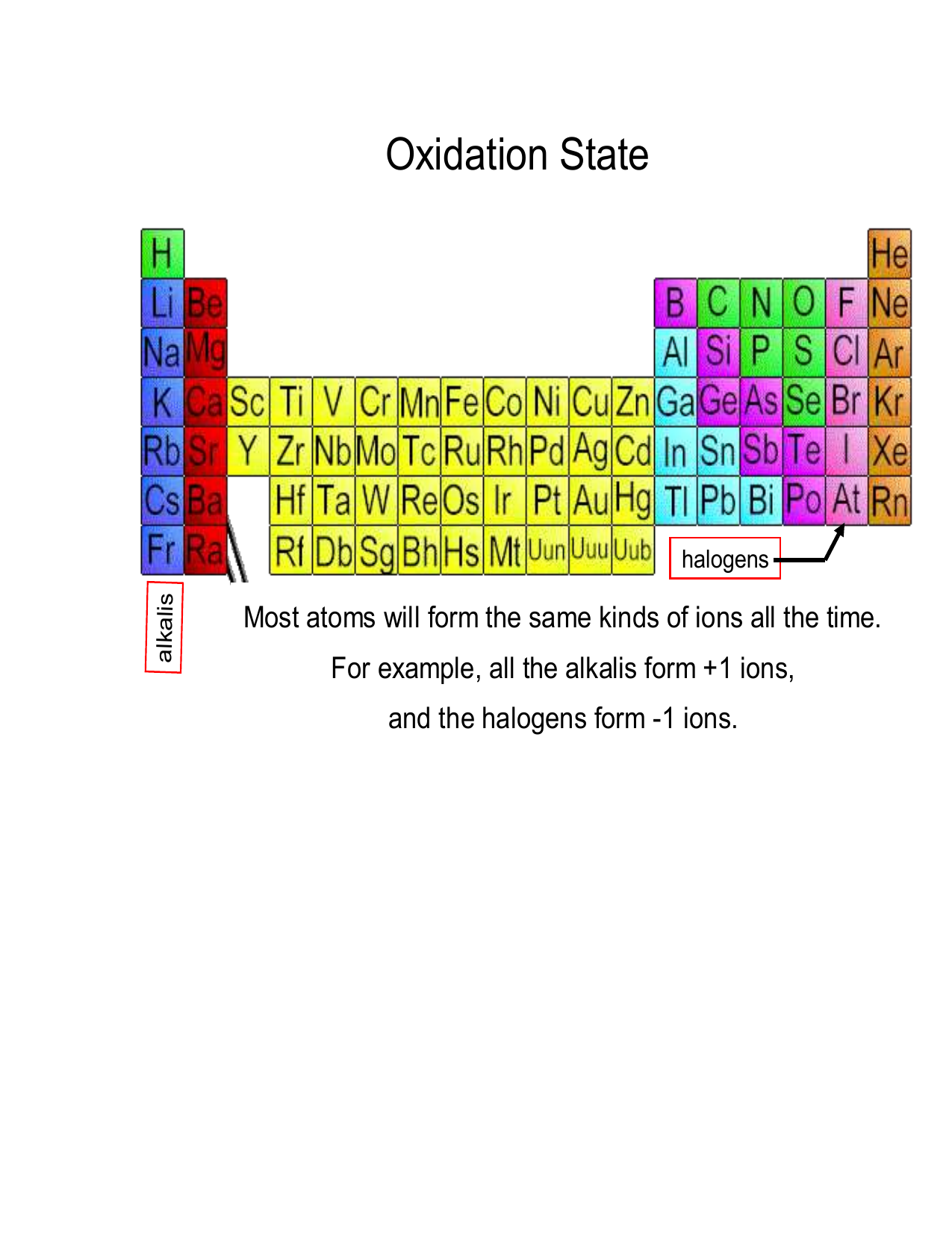
Chart Of Oxidation Number
Chart Of Oxidation Number - Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0. Kno 3 is a neutral compound. The sum of oxidation numbers of all the atoms in a. Oxidation number is 0 for atoms in an element. Web the atoms in he and n 2, for example, have oxidation numbers of 0. Bold numbers represent the more common oxidation states. Scandium is one of the two. Xe, cl 2, s 8,. That tells you that they.
Oxidation explained Science, Chemicalreactions, Chemistry, Periodic
That tells you that they. This color periodic table contains the number, symbol, name, atomic mass. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Kno 3 is a neutral compound. The oxidation number of a monatomic ion equals the charge of the ion.
Downloadable Periodic Table Oxidation States
Web todd helmenstine this periodic table contains the oxidation numbers of the elements. This applies regardless of the structure of the element: The sum of oxidation numbers in a neutral compound is 0. Oxidation number is 0 for atoms in an element. Web this browse explains what corrosion states (oxidation numbers) are and how to compute and use them.
65 INFO PERIODIC TABLE ELEMENTS OXIDATION NUMBERS FREE DOWNLOAD PDF DOC
The oxidation number of a monatomic ion equals the charge of the ion. Hydrogen exists as a diatomic gas in its elemental form. Ii) always form ionic bonding by either gaining or losing electrons, irrespective of the actual nature. The sum of oxidation numbers in a neutral compound is 0. The sum of oxidation numbers of all the atoms in.
The Periodic Table of Oxidation States Compound Interest
Web the atoms in he and n 2, for example, have oxidation numbers of 0. For monatomic ions, the oxidation number is the charge. Web the corrected table can be found here. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0. That tells you that they.
Oxidation Numbers
Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Hydrogen exists as a diatomic gas in its elemental form. Web the oxidation number of any atom in its elemental form is 0. This table also contains the element number, element symbol, element name and atomic weights of each.
PPT Elements and Periodic Table PowerPoint Presentation, free
Web the oxidation number of each hydrogen atom in most of its compounds is +1, except in metal hydrides (e.g., nah, lih) where it is. Values in italics represent theoretical or unconfirmed oxidation numbers. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0. Web charges given to atoms in a molecule.
Periodic Table Of Oxidation Numbers Gordon Fastir
This color periodic table contains the number, symbol, name, atomic mass. The oxidation number of a monatomic ion equals the charge of the ion. That tells you that they. Web todd helmenstine this periodic table contains the oxidation numbers of the elements. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0.
Labeled Periodic Table With Oxidation Numbers Periodic Table Timeline
Web this page explains what oxidation states (oxidation numbers) are and how to calculate additionally use them. Web the oxidation number of each hydrogen atom in most of its compounds is +1, except in metal hydrides (e.g., nah, lih) where it is. Values in italics represent theoretical or unconfirmed oxidation numbers. Web charges given to atoms in a molecule in.
Periodic Table Of Oxidation Numbers Gordon Fastir
Group 1a and 2a elements have the same oxidation number. Ii) always form ionic bonding by either gaining or losing electrons, irrespective of the actual nature. Xe, cl 2, s 8,. For monatomic ions, the oxidation number is the charge. What are the rules for oxidation numbers?
Common Oxidation Numbers Chart
Ii) always form ionic bonding by either gaining or losing electrons, irrespective of the actual nature. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0. Web todd helmenstine this periodic table contains the oxidation numbers of the elements. This color periodic table contains the number, symbol, name, atomic mass. Web this.
The sum of all oxidation numbers in a molecule or ion must add up to the. Bold numbers represent the more common oxidation states. Web the atoms in he and n 2, for example, have oxidation numbers of 0. Web the oxidation number of each hydrogen atom in most of its compounds is +1, except in metal hydrides (e.g., nah, lih) where it is. Web charges given to atoms in a molecule in this way are called oxidation numbers. Thus, the atoms in o2, o3, p4, s8, and aluminum metal all have an oxidation number of 0. Web the (ii) and (iii) are the oxidation states of the iron in the two compounds: Web todd helmenstine this periodic table contains the oxidation numbers of the elements. Web the following chart describes the most common oxidation states of the period 3 elements. Web this browse explains what corrosion states (oxidation numbers) are and how to compute and use them. Web this page explains what oxidation states (oxidation numbers) are and how to calculate additionally use them. That tells you that they. The sum of oxidation numbers in a neutral compound is 0. Web the oxidation number of a monatomic (composed of one atom) ion is the same as the charge of the ion. Values in italics represent theoretical or unconfirmed oxidation numbers. Web 60 rows oxidation number; Oxidation number is 0 for atoms in an element. Web i) bonds with heteroatoms. We can use oxidation numbers to keep track of. This table also contains the element number, element symbol, element name and atomic weights of each element.
Web This Browse Explains What Corrosion States (Oxidation Numbers) Are And How To Compute And Use Them.
Web this page explains what oxidation states (oxidation numbers) are and how to calculate additionally use them. The sum of all oxidation numbers in a molecule or ion must add up to the. Web 60 rows oxidation number; The sum of oxidation numbers of all the atoms in a.
Web The (Ii) And (Iii) Are The Oxidation States Of The Iron In The Two Compounds:
Web the oxidation number of any atom in its elemental form is 0. Web the following chart describes the most common oxidation states of the period 3 elements. Hydrogen exists as a diatomic gas in its elemental form. The sum of oxidation numbers in a neutral compound is 0.
That Tells You That They.
Group 1a and 2a elements have the same oxidation number. Bold numbers represent the more common oxidation states. Oxidation number is 0 for atoms in an element. This table also contains the element number, element symbol, element name and atomic weights of each element.
The Oxidation Number Of A Monatomic Ion Equals The Charge Of The Ion.
Web the corrected table can be found here. Web the atoms in he and n 2, for example, have oxidation numbers of 0. Web the oxidation number of each hydrogen atom in most of its compounds is +1, except in metal hydrides (e.g., nah, lih) where it is. This applies regardless of the structure of the element: